Use the word “battery” in conversation and most people will probably think of the batteries used to power torches, games and other small household items. But to an engineer, of course, batteries are devices that can store and release electrical energy and can come in all shapes and sizes.
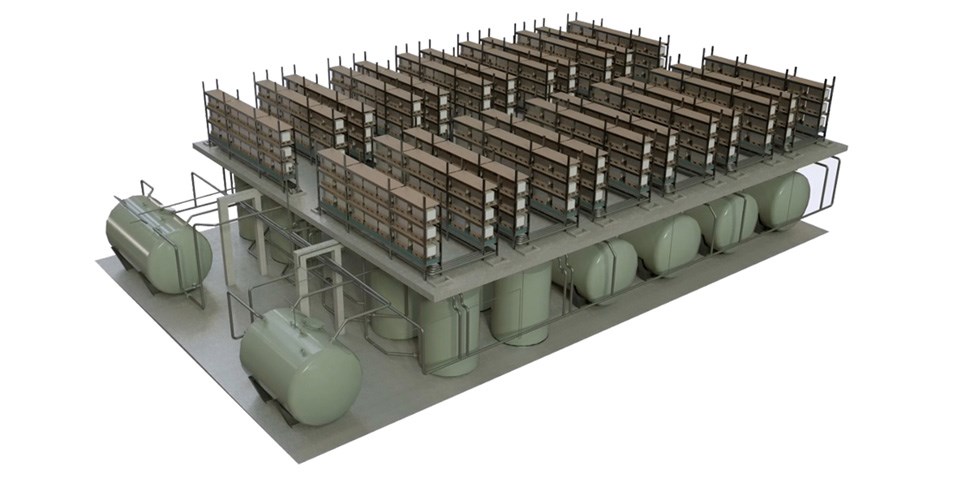
Researchers at the Fraunhofer Institute for Chemical Technology ICT, for example, are currently busy with so-called redox-flow batteries. Such systems are electrochemical energy storage devices that are based on a liquid storage medium. To give a sense of scale, practical redox-flow batteries can vary in size from say a large cupboard to a twenty-foot container or even bigger.
Discussing the whys and wherefores of this new technology, Peter Fischer, the Group Leader for Redox-Flow Batteries, comments that such batteries are efficient and have a longer service life than conventional batteries. And, as the energy is stored in external tanks, the battery capacity can be scaled independently of the rated battery power.

Basic principles
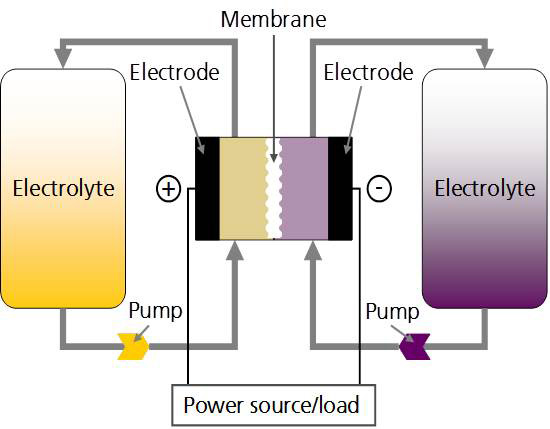
Mr Fischer further explains the underlying principles of the redox-flow batteries. “In the electrochemical cell, electrolyte solutions flow through the half-cell compartments of the plus and minus poles. To prevent the two solutions from mixing, the half-cells are separated by an ion-conducting or semi-permeable membrane. The potential difference of the electrolytes generates a voltage at the electrodes. If the electric circuit is closed, an electrochemical reaction sets in and electricity begins to flow. In order to charge the electrolytes, an external voltage is applied to the cell, and the reaction in the half-cells is initiated in the opposite direction. This reaction charges the electrolyte.”
As in fuel cells, individual cells can be combined in series to create a so-called “cell stack”. The stacks themselves are then connected fluidically and electrically to batteries.
Asked about the benefits and applications, Mr Fischer replies as follows: “as redox-flow batteries are based on external energy storage media, the power and capacity of the battery can be scaled independently: the volume of electrolyte determines the battery capacity (the “quantity” of energy stored), while the surface area and number of cells determines the power. The storage of electrolyte in separate tanks means that virtually no self-discharge occurs when the system is not in operation. This makes the technology suitable for application in uninterrupted power supply. A further field of application is the storage of energy from renewable sources, such as solar and wind.”
Materials development
An important challenge in the field of redox-flow batteries is to reduce the cost of the functional materials in the stack. Mr Fischer: “In order to achieve a significant cost reduction while at the same time optimizing the electrochemistry of the system, we are continuously investigating new materials in terms of their potential for application in redox-flow batteries. For this purpose individual cells are set up and characterized using electrochemical methods.”
In the cell test, materials such as membranes, porous and textile electrodes, materials for bipolar plates, alternative cell geometries and novel electrolyte formulations are tested regarding their performance and efficiency.
Mr Fischer also discussed suitable materials for the valves used in redox flow batteries. This is apparently quite a challenging area, given that the solutions can be quite corrosive for certain polymers or elastomers. For more details on this topic, please read his interview in the September 2016 Valve World. Not a subscriber? Please contact
d.sear@kci-world.com for a PDF copy.