Accurate measurement of hydrogen sulphide (H2S) in natural gas is critical for safety, compliance and operational performance. Now that liquid detection systems such as LineVu are detecting the presence of liquid carryover in natural gas streams at considerably higher levels than expected, this paper examines the partitioning behaviour of H2S between gas and liquid phases in the presence of condensate using Henry’s Law. A quantitative model is developed to show how increasing liquid volume fraction (LVF) leads to significant underreporting of gas-phase H2S, potentially misleading gas quality assessments.
By Paul Stockwell, Process Vision
Hydrogen sulphide (H2S) is a toxic, corrosive and regulated component of natural gas. Monitoring its concentration with precision is vital for meeting pipeline specifications and environmental standards. H2S-specific analysers are commonly used for this purpose. However, these instruments are gas phase measurements and assume that the gas is dry. When liquids are present in the pipeline, H2S can partition into the liquid phase, leading to erroneous gas-phase measurements.
This paper explores the effect of condensate on H2S partitioning, quantifies the error introduced in H2S readings and offers guidance on how to mitigate this problem. It is acknowledged that ideal gas law may not apply to natural gas calculations. For the purposes of this paper, the differences caused by the compressibility factor of natural gas are minimal and so have been ignored as the example calculations are for illustrative purposes only.
Theoretical background
Henry’s Law describes the equilibrium between gas and liquid phases for a dissolvable gas:
C = kH P
Where: C = Concentration of gas in the liquid phase (mol/L)
kH = Henry’s Law constant (mol/L·atm)
P = Partial pressure of the gas (atm)
H2S is highly soluble in water and moderately soluble in hydrocarbon liquids. The Henry’s Law constant for H2S in water at 25°C (77°F) is approximately 0.087 mol/(L·atm)1. In hydrocarbon condensates, the value is typically estimated between 1.0 to 3.0 mol/(L·atm)1, depending on composition and temperature2.
Methodology
To model the impact of LVF on gas-phase H2S readings, we consider a standard case:
Total H2S: 5 ppm (mole fraction)
Pressure: 1000 psi (68.95 atm)
Temperature: 25°C (298 K)
LVF: 0% to 1% (0 to 0.01 L of liquid per litre of gas)
Using ideal gas behaviour, the total moles of gas and H2S per litre are calculated. Partitioning is then modelled using Henry’s Law, assuming equilibrium between gas and liquid.
Results and discussion
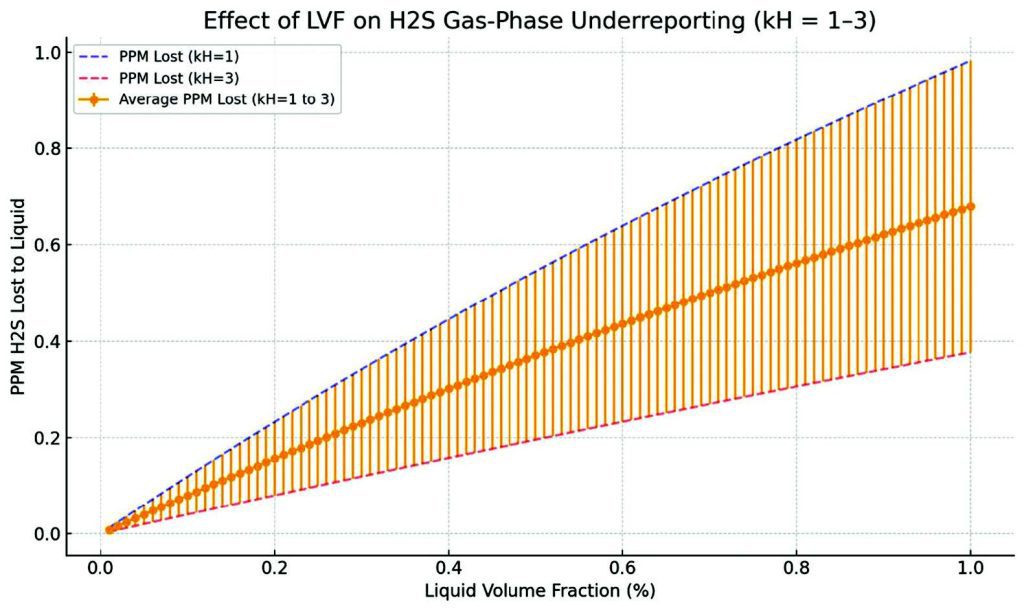
The calculations show that even a 0.1% LVF results in approximately 1.6% underreporting of H2S concentration. At 0.5% LVF, this error increases to more than 7%. This is significant, especially near threshold limits for pipeline specification (e.g., 2 ppm for transmission). An Excel tool has been developed (see Appendix) to allow operators to input local conditions and quantify the potential measurement error based on LVF.
Key observations:
- Higher pressure increases the partial pressure of H2S, increasing partitioning into liquid.
- Higher LVF results in a larger sink for soluble gas species.
- Under-reporting is systematic and repeatable, making it predictable but dangerous.
Practical implications: Compliance risk
Gas reported as compliant may exceed H2S thresholds after phase separation. Corrosion risk: Underestimating H2S can lead to under-injection of scavengers and corrosion inhibitors. Measurement inaccuracy: This effect undermines the reliability of all H2S measurement systems as it is a physical effect locking H2S up in liquids that may be either in a mist flow or stratified flow. In all gas analysis systems at custody transfer points, these liquids are either:
a) Avoided, in the case of stratified flow, by sampling according to API 14.15 using a sample probe to sample from the middle of the pipeline or;
b) Removed by a coalescing or membrane filter before the gas phase sample arrives at the gas analyser.
c) The uncertainty budget for H2S analysis should include the baseline accuracy of the measurement systems, errors introduced by temperature and pressure changes in the sample system and, unless a pipeline liquid detection system is installed, the possibility
of unknown liquids being present in the gas stream should be included in the uncertainty statement (accuracy) for the analysis. This is because pipeline liquid detection systems such as LineVu have demonstrated that calculated and measured hydrocarbon dewpoint systems do not guarantee that liquid hydrocarbons are not present in the pipeline.
Mitigation strategies: Liquid carryover is considered a fault condition. It affects the accuracy of flow measurement, gas analysis systems and increases the risk of corrosion, compressor trips and compressor failures. Introducing a liquid carryover detection system (e.g., optical or visual monitoring) upstream of analysers, alerts operators to this fault condition.
Conclusion
The presence of even small volumes of liquid in a natural gas stream introduces a measurable and significant bias in gas-phase H2S concentration readings. This is governed by well-understood thermodynamics, notably Henry’s Law. Operators and measurement engineers must consider LVF as a critical factor in gas quality assurance.
Appendix
If you would like the full paper, you can email jo.shailes@processvision.com.
Results and discussion
1: Sander, R. (2015). “Compilation of Henry’s Law Constants”.
2: Atmospheric Chemistry and Physics. – Gmehling, J., Rasmussen, P., et al. (1994). “Vapor-Liquid Equilibrium by UNIFAC Group Contribution”.
3: Industrial & Engineering Chemistry Research. – NIST Chemistry WebBook. (https://webbook.nist.gov?utm_ source=chatgpt.com)
4: GPA Midstream Standard 2261: Gas Analysis and Reporting Guidelines (2023 Edition)
5: API 14.1
About this Technical Story
This Technical Story is an article from our Valve World Magazine, September 2025 issue. To read other featured stories and many more articles, subscribe to our print magazine. Available in both print and digital formats. DIGITAL MAGAZINE SUBSCRIPTIONS ARE NOW FREE.
“Every week we share a new Technical Story with our Valve World community. Join us and let’s share your Featured Story on Valve World online and in print.”

